For all you non-scientists out there who are currently inwardly groaning at the mere mention of chemistry, I’m here to make it simple (or at least a bit simpler).
You don’t really need to know all this to follow a soap making recipe but it really helps if you would like to design your own recipes later. I always like to know what is going on in a chemical reaction because that way it can be controlled. And that means there is less chance of something going wrong that you can’t explain.
What is soap and how does it work?
First, we need to understand exactly what soap making is. In a nutshell, it is the chemical reaction between a sodium or potassium hydroxide with a triglyceride (fat). This reaction is known as saponification.
We want to make soap because it is a surfactant. Soap helps to hold oil particles in suspension with water in much the same way as egg yolk does with the oil in mayonnaise. In effect, a surfactant is an emulsifier. It helps oil and water to mix.
We need surfactants to help water clean our clothes and bodies because water is not very efficient at cleaning on its own. Water has a high surface tension which allows it to form droplets instead of spreading out when it hits a surface. It also does not dissolve oil well and this is a problem because the oil particles are where the dirt usually is.
So how does soap help?
It is down to the structure of the soap molecule.
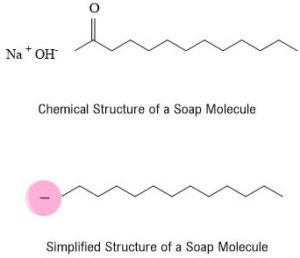
There are two distinct areas in this molecule, a negatively charged head and a long tail which is uncharged. On one end there is a negatively charged head which is hydrophilic (mixes with water). On the other, there is a long chain tail which does not have a charge and this is hydrophobic (doesn’t mix with water).
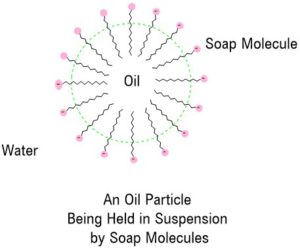
Having a molecule which has water-loving and water-hating ends allows the soap molecule to form a bridge between each of the oil and water layers.
The hydrophilic charged end mixes with the water and the hydrophobic end mixes with the oil. Eventually, enough soap molecules combine with the oil and water that they hold the oil in suspension within the water.
These spherical complexes are known as micelles. In this way, the oil particles can be separated from the surface, held in suspension and then washed away.
Soap is an anionic surfactant and other detergents such as SLS are cationic surfactants as they have a positively charged head.
The reactants of saponification
The Sodium Hydroxide (lye)
In our case, we are using NaOH (lye) as the hydroxide. Once this is dissolved in water, it separates into two parts, a Sodium ion and a Hydroxide ion. We call this a strong base because it almost totally breaks down into its constituent ions. It is really very reactive.
In order to pull the positively and negatively charged ions apart, energy must be added. This is called the Lattice Energy. As the water molecules are attracted to and surround the ions, energy is released into the solution. This is called the Hydration Energy. The hydration energy released is more than the lattice energy taken in to break the bonds and that is why the solution heats up. It is an exothermic reaction.
The Fats
Fats or triglycerides are made up of fatty acids and glycerol
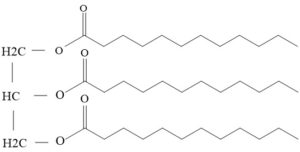
The fatty acids are the part that combines with the Sodium to make up the soap molecule. Fatty acids come in two types depending on the structure of the carbon-based tail. Saturated fatty acids (like lauric and myristic acids) have a long straight chain whereas unsaturated fatty acids (like oleic and linoleic acids) have a bend in their chain due to a double bond.
Each Triglyceride contains a glycerol molecule with three fatty acid tails.
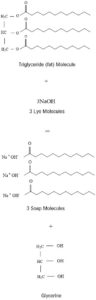
In the saponification reaction, the glycerol side of the molecule splits off and forms glycerine. This leaves 3 fatty acid tails. Each of these reacts with a Sodium ion and forms a soap molecule (which is actually the salt of the fatty acid).
Each fatty acid requires one Sodium Ion to react with it so each molecule of fat needs three sodium ions.
The different fatty acid tails are different weights. You get more molecules in one gram of an oil with shorter (and lighter) chain fatty acids and fewer molecules in a gram if the fatty acid chains are longer (and heavier).
Scientists have worked out the proportions of lye (NaOH)required for each oil, depending on the fatty acid composition of the oil. These are called SAP values.
This way we can ensure that each molecule of lye is reacted completely with an oil molecule and there are no free lye molecules left in the soap.
We can test for free lye molecules in the soap we make using phenolphthalein. If there are no free OH- ions, the soap will remain colourless. If there are free OH- ions, the soap will turn a vibrant bright pink.
So there you go…It wasn’t that bad really! As I said at the start, you really don’t need to know all this if you are just following a simple recipe but it does come in handy if you are formulating your own recipes.


